Citric Acid Pka Values
11 10 2. These are all multiple choice.

Mind The Buffering Capacity Of Citric Acid
Strong bases completely dissociate in aq solution Kb 1 pKb 1.
. Compiled from Appendix 5 Chem 1A B C Lab Manual and. What is the predominant form of citric. Many microbial cultures are buffered with citric acid over a pH range of 25 to 70 since the pKa values for this triprotic acid are 313 476 and 640 as shown in The Merck Index pp 330-331.
C 6 H 8 O 7. PKa values are 521 428 and 292 at 25 C extrapolated to zero ionic strength. Relating pH Ka and pKa pH Ka.
Oxalic acid CO 2 H 2. On the other hand Hofer 2015 stated that citric acid has three distinct pKa values which are pH 3 pH 4 and pH 6. Conjugate acids cations of strong bases are ineffective bases.
D-Tartaric Acid C 4 H 6 O 6 30. CHCl 2 CO 2 H. 45 rows Chlorous acid.
This acid is also an intermediate in the metabolism of several aerobic organisms since it participates in the tricarboxylic acid cycle. Citric acid is found in fruits such as lemons and oranges. Citric acid anhydrous is produced from hot saturated solutions of citric acid.
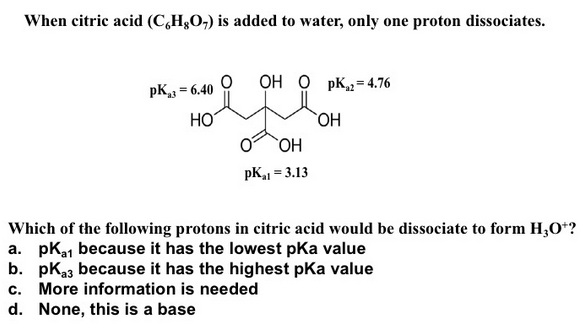
In order to confirm the ionisation of the hydroxyl group of citric acid at pH values lower than 12 pKa 116 Migal and Sychen 1958 a potentiometric titration was carried out. PKa is a value that indicates how weak or powerful an acid is in simple terms. In view of its three carboxylic acid functional groups it has three pKa values at pH 31 47 and 64.
Phthalic Acid C. The value Kₐ lies around 10 - 5 105 for carboxylic acids and hence the pKₐ value of carboxylic acids is around 5. The pKa values obtained from the titration two with the use of citric acid are.
17 10 5. H 2 CrO 4. The pK a value is used to choose a buffer when needed.
8 H 6 O 4 29 54 19-64. Citric acid is a tricarboxylic acid with a molecular weight of 21014 Da. BH B H The pKb for a base may be calculated from the pKa value of its conjugate acid.
What is the predominant form of citric acid at pH 449. For example the pKₐ value of formic acid is 375 while the pKₐ. Citric acid is a good buffering agent for solutions between about pH 2 and pH 8.
What is the predominant form of citric acid H3C6H5O7 at pH 437 and pH 612. Citric acid H3C6H507 has pKa values of 3128 pKa1 4761 pKa2 and 6396 pKa3. For bases the pka value is given for the conjugate bases BH and BH22.
3-phosphoglyceric acid 142 342 54 C6H5CH2- 23 755 57 2-phosphoglyceric acid 142 355 71 NH3CH24- 255 755 57 peroxymonophosphoric acid 405 69 NH3CH25- 26 76. PKa is the negative log base ten of the Ka value to be precise acid. CCl 3 CO 2 H.
Citric acid is a weak tricarboxylic acid found in citrus fruits like lemons which contain 79 citric. 40 10 7. 18 10 1.
Monochloroacetic Acid C. Choosing an acid or base where pK a is close to the pH needed gives the best results. 32 10 7.
Citric acid has pKa values of 3128 pKa1 4761pKa2 and 6396 pKa3. 74 10 4. Chloroacetic Acid Sodium Salt.
PKw pKa pKb At. The pKa of a strong acid is less than zero. K 1 65 10-2 K 2 61 10-5.

Stepwise Deprotonation Of Citric Acid Hcith 3 With The Respective Pk Download Scientific Diagram

A Chemical Structure And P Ka Values Of Citric Acid B Download Scientific Diagram

Table 1 From Citric Acid Adsorption On Tio2 Nanoparticles In Aqueous Suspensions At Acidic And Circumneutral Ph Surface Coverage Surface Speciation And Its Impact On Nanoparticle Nanoparticle Interactions Semantic Scholar

Organic Chemistry How Will Citric Acid Be Deprotonated By Pyridine Chemistry Stack Exchange
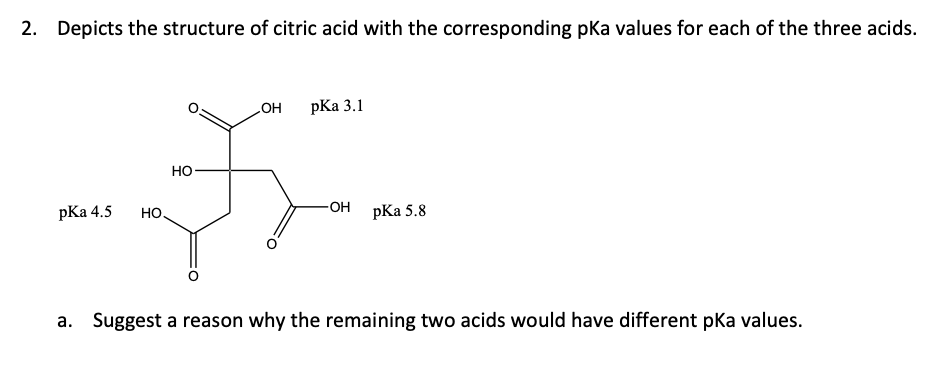
Solved 2 Depicts The Structure Of Citric Acid With The Chegg Com

Solved When Citric Acid C Hg0 Is Added To Water Only One Chegg Com
Comments
Post a Comment